This is an ion. An ion is a charged atom or molecule.It is charged because the number of electrons do not equal the number of protons in the atom or molecule. An atom can acquire a positive charge or a negative charge depending on whether the number of electrons in an atom is more or less then the number of protons in the atom.
When an atom is attracted to another atom because it has an non equal number of electrons and protons, the atom is called an ION. If the atom has more electrons than protons, it is a negative ion, or ANION. If it has more protons than electrons,it is a positive ion.
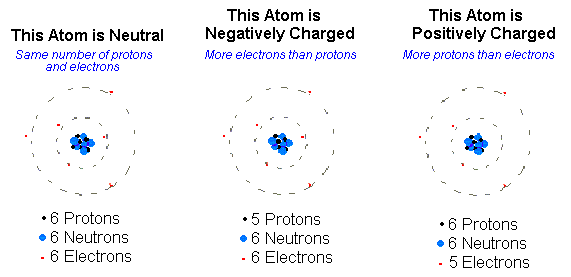
The outer shell of any atom is called the valence shell. When the valence electron in any atom gains common energy from some outside force, it can break away from the parent atom and become what is called a free electron.They can also be positive because they like to loose electrons, or negitive because they like to gain electrons.

An ionic bond is a type of chemicial bond formed through an electric between two diffrently charged ions. Ionic bonds are formed between a cation, which is usually a metal, and an anion, which is usually nonmetal.

well stay cationios, and ion to see you later. stay positive and or negitive.
Corny puns rule!
You did really good for your second blog! There were some spelling errors,but overall good job!
ReplyDelete